The Cupid Peptide Company
EXPERTS IN CELL PENETRATING PEPTIDE
CUSTOM DESIGN AND MANUFACTURE
What is Cupid?
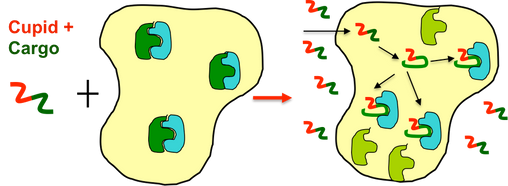
Cupid is a proprietary cell penetrating peptide developed by the Cupid Peptide Company. It is able to pass through the lipid bilayer membranes of living cells. Our company uses Cupid as a vehicle to deliver bioactive peptide ‘Cargoes’ to the interior of living cells in order that the interactions of the peptide with the cells protein machinery may be more easily studied.
We have optimized the production process to attach Cupid to long peptide sequences (200+ amino acid “Cargoes”) and are now able to offer researchers a variety of Cupid-linked products capable of being delivered efficiently to the interior of living cells. Our studies with fluorescently labelled Cupid Products and the Green Fluorescent Protein (See Proof of Principle with GFP) demonstrate that each is delivered in a diffuse pattern across all structures in the cell.
Demonstrating Cupid Is Cell Penetrating
To demonstrate Cupids ability to penetrate cells we labelled the Cupid Control peptide with fluorescein using a commercially available labelling kit.
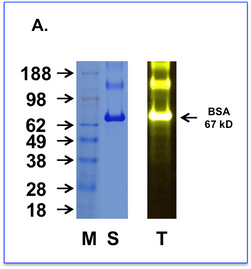
As a negative control we chose a commonly available protein, Bovine Serum Albumin to compare with Cupid, which we also labelled with fluorescein (Figure A.).
Cupid Peptides FITC labeled BSA
A. Bovine Serum Albumin (BSA) from a commercial source was labelled with fluorescein and subjected to SDS-PAGE alongside a prestained molecular marker ladder. The gel was then stained for protein with a commercial coomassie-based stain (Left Panel) or observed with a blue light transilluminator (Right Panel)
M = Weight markers shown in kD
S = Protein stained BSA sample
T = Transilluminated Fluorescein labelled BSA sample
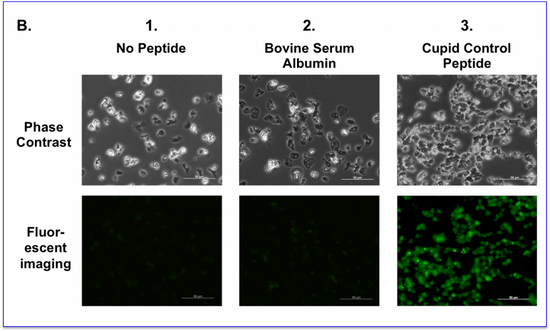
Using the peak absorbance at 495 nm to calculate the fluorescein incorporation, we incubated amoeba cells with equivalent amounts (10 micromolar) of labelled Cupid Control peptide or BSA for 60 minutes. After washing and mounting, the cells were imaged with phase contrast and fluorescent microscopy and compared with the autofluorescence of untreated cells (Figure B.).
B. Living amoeba cells were either
(1) left untreated
(2) incubated with FITC labelled BSA at 10 micromolar
(3) incubated with FITC labelled Cupid Control peptide at 10 micromolar
After 60 minutes the media was exchanged to wash the peptide away. Cells were then imaged using phase contrast (Upper Panel) or filter sets for Fluorescein (Lower Panel).
Result:
At a setting where the background autofluorescence of the untreated cells is at the threshold of detection, there was no significant uptake of fluorescence in untreated or FITC-BSA treated cells, whereas we observe the FITC-Cupid Control peptide fluorescence distributed diffusely throughout all cells.
Conclusion:
Cupid Control peptide is cell permeable whereas BSA is not cell permeable.
A Brief Overview of Cell Permeable Peptides (CPP)
We shall define a CPP, a cell penetrating peptide (also referred to as cell permeable) as a peptide that can get through the cell outer membrane and enter into cells. We exclude from our definition peptides, proteins and other reagents that form pores or punch holes in the membrane itself. In our view there are 3 main classes of such CPPs.
The classes are:-
1) Basic charged (+) amino acids, commonly a string of Arginine or Lysine residues
2) Viral peptides, of which the TAT sequence from HIV is the most studied.
3) Membrane permeable peptides, such as “Cupid”.
The first 2 classes do not, in some senses, truly permeate the membrane but effect entry into the cell by being incorporated into vesicles taken within the cell during the dynamic processes of membrane recycling / receptor internalisation.
Unlike the first two classes, the third class of cell permeable peptide is capable of ‘true’ permeation through cell membranes by insinuating themselves into a lipid bi-layer in a reversible manner. Although the physics of the mechanism is poorly understood, this characteristic allows them to transverse biological and even artificial membranes without requiring a surface receptor or cellular (ATP) energy (1-7).
References
1) Alain Prochiantz (2000) Messenger proteins: homeoproteins, TAT and others Article. Curr. Opin Cell Biol., 12, 400-406.
http://www.sciencedirect.com/science/article/pii/S0955067400001083
2) Xanthi Antoniou and Tiziana Borsello (2010) Cell Permeable Peptides: A Promising Tool to Deliver Neuroprotective Agents in the Brain. Pharmaceuticals, 3, 379-392.
Pdf = http://www.mdpi.com/1424-8247/3/2/379/pdf
3) Ofelia Maniti, Isabel Alves, Germain Trugnan, Jesus Ayala-Sanmartin (2010) Distinct Behaviour of the Homeodomain Derived Cell Penetrating Peptide Penetratin in Interaction with Different Phospholipids. PloS ONE, 5(12), e15819.
http://www.plosone.org/article/info%3adoi/10.1371/journal.pone.0015819
4) Richard, J. P., Melikov, K., Vives, E., Ramos, C., Verbeure, B., Gait, M. J., Chernomordik, L. V., and Lebleu, B. (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem 278, 585–90.
http://www.ncbi.nlm.nih.gov/pubmed/12411431
5) Paulo Almeida and Antje Pokorny (2009) Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry 48, 8083–93.
http://www.ncbi.nlm.nih.gov/pubmed/19655791
6) Duchardt, F., Fotin-Mleczek, M., Schwarz, H., Fischer, R., and Brock, R. (2007) A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 8, 848–66.
http://www.ncbi.nlm.nih.gov/pubmed/17587406
7) Jones, S. W., Christison, R., Bundell, K., Voyce, C. J., Brockbank, S. M., Newham, P., and Lindsay, M. A. (2005) Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol 145, 1093–102.