The Cupid Peptide Company
EXPERTS IN CELL PENETRATING PEPTIDE
CUSTOM DESIGN AND MANUFACTURE
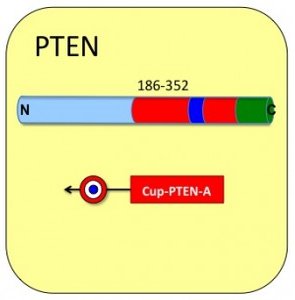
Cupid-PTEN-A
Product Description:
Cupid-PTEN-A peptide:
• Cargo: Residues 186 to 352 of human PTEN
• Domain type: C2 Region. Plasma membrane interaction and p53 binding region.
• 167 Amino acids
LDYRPVALLFHKMMFETIPMFSGGT
CNPQFVVCQLKVKIYSSNSGPTRRE
DKFMYFEFPQPLPVCGDIKVEFFHK
QNKMLKKDKMFHFWVNTFFIPGPEE
TSEKVENGSLCDQEIDSICSIERADN
DKEYLVLTLTKNDLDKANKDKANRY
FSPNFKVKLYFTKTVE
Product Characteristics:
• Molecular weight (daltons): 27208
• Isoelectric point (pI): 8.77
• Extinction Coefficient (A280 reduced) : 21430
• Solubility : >200 micromolar
• Purity: 85%
• Cell-Permeation : Passes
• 1 Unit = 10 nanomoles = 0.272 mg
Cupid-PTEN-A Peptide Data
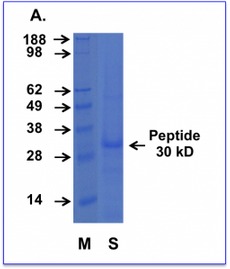
Cell permeating Cupid PTEN peptide AA. Purified Cupid-PTEN-A peptide was subjected to SDS-PAGE alongside a prestained molecular marker ladder. The gel was then stained for protein with a commercial coomassie-based stain.
M = Weight markers shown in kD
S = Cupid-PTEN-A peptide sample. The protein runs slightly anomalously appearing at 30 kD
Cell permeating Cupid
PTEN peptide A
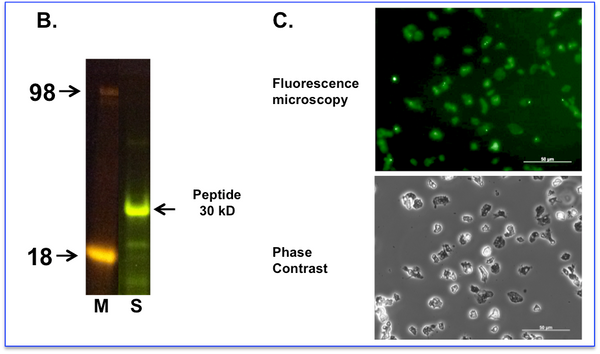
B. Cupid-PTEN-A peptide labelled with fluorescein was subjected to SDS-PAGE and observed with a blue light transilluminator.
M = Weight markers shown in Kd
S = Labelled Cupid-PTEN-A peptide sample.
C. Labelled peptide was incubated with living cells at 10 micromolar for 60 minutes before exchanging the media and washing the cells. Cells were imaged using a fluorescence microscope with filter sets for Fluorescein (Upper Panel) or phase contrast (Lower Panel). Fluorescent images of treated cells are taken at a setting where the background (autofluorescence) of the untreated cells is at the threshold of detection. We observe the peptide fluorescence distributed diffusely throughout all cells.
Comment: PTENs mass is actually only 47 kD but it runs as a 55-kilodalton (kD) protein on SDS-PAGE. This difference may be due to the high acidity of part of the protein, slowing its migration on SDS-PAGE, thus making it appear heavier than it actually is (1). The Cupid-PTEN-A peptide contains the acidic region within it which we suggest causes the slightly anomalous migration pattern seen in our laboratory.
(1) Reference.
The anomalous electrophoretic behavior of the human papillomavirus type 16 E7 protein is due to the high content of acidic amino acid residues.
Armstrong DJ, Roman A., Biochem Biophys Res Commun. 1993 May 14;192(3):1380-7.