The Cupid Peptide Company
EXPERTS IN CELL PENETRATING PEPTIDE
CUSTOM DESIGN AND MANUFACTURE
PTEN
PTEN (Phosphatase and Tensin Homologue Deleted from Chromosome-10) has been identified as one of the most frequently mutated tumor suppressors in human cancer. It functions primarily as a cytoplasmic phosphatase to regulate crucial signal transduction pathways involving growth, adhesion, migration, invasion and apoptosis. It has been demonstrated to be involved in the regulation of a variety of phenotypes whereas its major function of tumour suppression is achieved by the downregulation of the oncogenic Akt/PKB (Protein Kinase-B) pathway. PTEN is expressed throughout the embryo, and has critically important functions in early embryonic development (1-5).
PTEN has both protein phosphatase and an inositol phospholipid phosphatase enzymatic activity. PTENs predominant enzymatic activity is dephosphorylation of Inositol Phospholipids (phosphoinositides): PI(3,4,5)P3 (Phosphatidylinositol 3,4,5-trisphosphate or PIP3) and PI(3,4)P2 (Phosphatidylinositol 3,4-bisphosphate or PIP2) at the D3 positions into PI(4,5)P2 (Phosphatidylinositol 4,5-bisphosphate) and PI(4)P (Phosphatidyl Inositol Phosphate or PIP) respectively, thus antagonizing the PI3K (Phosphatidylinositiol-3 Kinase) activation (6).
PTEN also dephosphorylates protein substrates such as FAK (Focal Adhesion Kinase), the SHC-adapter protein, and Drebrin (Developmentally regulated Brain protein DBN) (7).
PTEN consists of a phosphatase domain that has a structure resembling protein tyrosine phosphatases but containing an enlarged active site that accounts for its ability to bind its PI (Phosphatidyl Inositol) substrates. There is a second major domain, termed C2, that binds phospholipids. This C2 domain appears to bind PTEN to the plasma membrane, and it might orient the catalytic domain appropriately for interactions with phosphatidylinositols and other potential substrates (8). Mutagenesis studies demonstrate the C-terminal tail of PTEN to be intriguing regulatory features that induce phosphorylation of certain serine and threonine residues (S380, T382 and T383) and can modulate both the enzymatic activity and the stability of PTEN. Loss of PTEN function, either in murine ES (Embryonic Stem) cells or in human cancer cell lines, results in accumulation of PI(3,4,5)P3 and PI(3,4)P2 and the subsequent activation of its downstream effectors, such as PDK-1 (Phosphoinositide-Dependent Kiinase-1), Akt, Btk family tyrosine kinases, PKC (Protein Kinase-C), and GEFs (Guanine Nucleotide Exchange Factors) for the Rho family of small GTPases (Guanosine Triphosphatases): Rac1 and CDC42. PTEN is capable of inducing apoptosis or a cell cycle arrest, and loss of PTEN in primary cells leads to either excessive proliferation or defects in apoptosis (9).
Cupid Cargo PTEN-A
LDYPRVALLFHKMMFETIPMFSGGTCNPQFVVCQLKVKIYSSNSGPTRREDKFMYFEFPQPLPVCGDIKVEFFHKQNKMLKKDKMFHFWVNTFFIPGPEETSEKVENGSLCDQEIDSICSIERADNDKEYLVLTLTKNDLDKANKDANRYFSPNFKVKLYFTKTVE
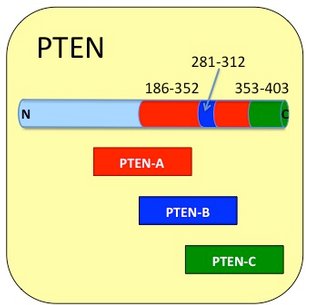
Amino acids 186 to 352 (167 amino acids)
C2 Motif Plasma membrane interaction and p53 binding region.
Cupid Cargo PTEN-B
PEETSEKVENGSLCDQEIDSICSIERADND
Amino acids 281 to 312. (32 amino acids)
“D-Loop” within the C2 motif
Reviews
1) The Protein-Protein interaction-mediated inactivation of PTEN. De Melo J, He L, Tang D. Curr Mol Med. 2013 Nov 17.
(2) The functions and regulation of the PTEN tumour suppressor. Song MS, Salmena L, Pandolfi PP. Nat Rev Mol Cell Biol. 2012 Apr 4;13(5):283-96. doi: 10.1038/nrm3330.
(3) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Cully M, You H, Levine AJ, Mak TW. Nat Rev Cancer. 2006 Mar;6(3):184-92. Review.
(4) The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Steelman LS, Bertrand FE, McCubrey JA. Expert Opin Ther Targets. 2004 Dec;8(6):537-50.
(5) The developmental biology of brain tumors. Wechsler-Reya R, Scott MP. Annu Rev Neurosci. 2001;24:385-428. Review.
(6) Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Science. 2002 Mar 15;295(5562):2088-91. Epub 2002 Feb 28.
(7) Phosphorylation of the actin binding protein Drebrin at S647 is regulated by neuronal activity and PTEN. Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, Parsons M, Eickholt BJ. PLoS One. 2013 Aug 5;8(8):e71957. doi: 10.1371/journal.pone.0071957. Print 2013.
(8) Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. Yamada KM, Araki M. J Cell Sci. 2001 Jul;114(Pt 13):2375-82. Review.
(9) Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Mol Cell Biol. 2000 Dec;20(23):8969-82.
PTEN interactions
PTEN contains a number of domains, including the N-terminal phosphoinositide binding motif and catalytic region, a C2 domain, two proline-, glutamic acid-, serine-, and threonine-rich segments and a PDZ-binding site at the C-terminal.
The crystal structure of PTEN (lacking the C-terminal tail) reveals intramolecular interaction between the N-terminal phosphatase and the C2 domain. The C2 domain of PTEN (residues 186-351) has been implicated in mediating membrane association of PTEN where it can act on inositol phospholipid substrates. The C2 region, however, appears to have a range of other functions such as a Pten - P53 interaction. Experiments using GST fusion proteins show that the C2 domain of PTEN interacts with the C-terminal regulatory domain of p53 (p53 residues 363–393) and enhances p53 transcriptional activity especially through its DNA binding activity (2). The C2 domain of PTEN also interacts with the Major Vault Protein (MVP) through and the first two EF hand domains of MVP (3). This region is contained in Product: Cupid-PTEN-A.
Within the C2 region there is another site termed the ‘d-Loop’ (residues 281 to 312) which has been recently shown to interact with actin binding protein Drebrin (Developmentally regulated Brain protein DBN) (4). The interaction causes dephosphorylation of DBN by PTEN and is inhibited by a cell permeating d-Loop peptide now available from this laboratory. This region is contained in Product: Cupid-PTEN-B.
The C-terminal ‘tail’ of PTEN interacts with several PDZ domain-containing proteins such as hDLG, hMAST205, MAGI-2, and MAGI-3. Peptides derived from the PTEN ‘tail’ or deletion of the carboxyl-terminal three amino acids of PTEN itself are capable of inhibiting the interaction with MAGI-3 (5). However the PDZ-binding site of PTEN seems not required for tumour suppression or other biological activities. The 71 amino acid C-Terminal region has also been found to interact within the C2 region and with the N-terminal phosphatase of PTEN itself, suggesting a complicated interplay exists between the different regions modulating auto-inhibition of phosphatase activity and of membrane translocation (6, 7). This region is Coming Soon.
The importance of C-Terminal interactions of PTEN in its biological function remains an area of great interest and the complete spectrum of PTEN-interacting proteins and the effects of the interactions on PTEN function are not yet defined.
Cupid Cargo PTEN-C
EPSNPEASSSTSVTPDVSDNEODHYRYSDTTDSDPENEPFDEDQHTQITKV
Amino acids 353 to 403. (51 amino acids)
C-Terminal “Tail” and PDZ-binding domain.
References
(1) http://en.wikipedia.org/wiki/PTEN_(gene)
(2) PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. Cancer Cell. 2003 Feb;3(2):117-30.
(3) PTEN associates with the vault particles in HeLa cells. Yu Z, Fotouhi-Ardakani N, Wu L, Maoui M, Wang S, Banville D, Shen SH. J Biol Chem. 2002 Oct 25;277(43):40247-52. Epub 2002 Aug 11.
(4) Phosphorylation of the actin binding protein Drebrin at S647 is regulated by neuronal activity and PTEN. Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, Parsons M, Eickholt BJ. PLoS One. 2013 Aug 5;8(8):e71957. doi: 10.1371/journal.pone.0071957. Print 2013.
(5) Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, Lasky LA. J Biol Chem. 2000 Jul 14;275(28):21477-85.
(6) Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Science. 2004 Feb 20;303(5661):1179-81.
(7) Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. Odriozola L, Singh G, Hoang T, Chan AM. J Biol Chem. 2007 Aug 10;282(32):23306-15. Epub 2007 Jun 12.